Startup to Apply For Approval to Produce Heart Cell Sheets from iPS Cells for Heart Disease Treatment
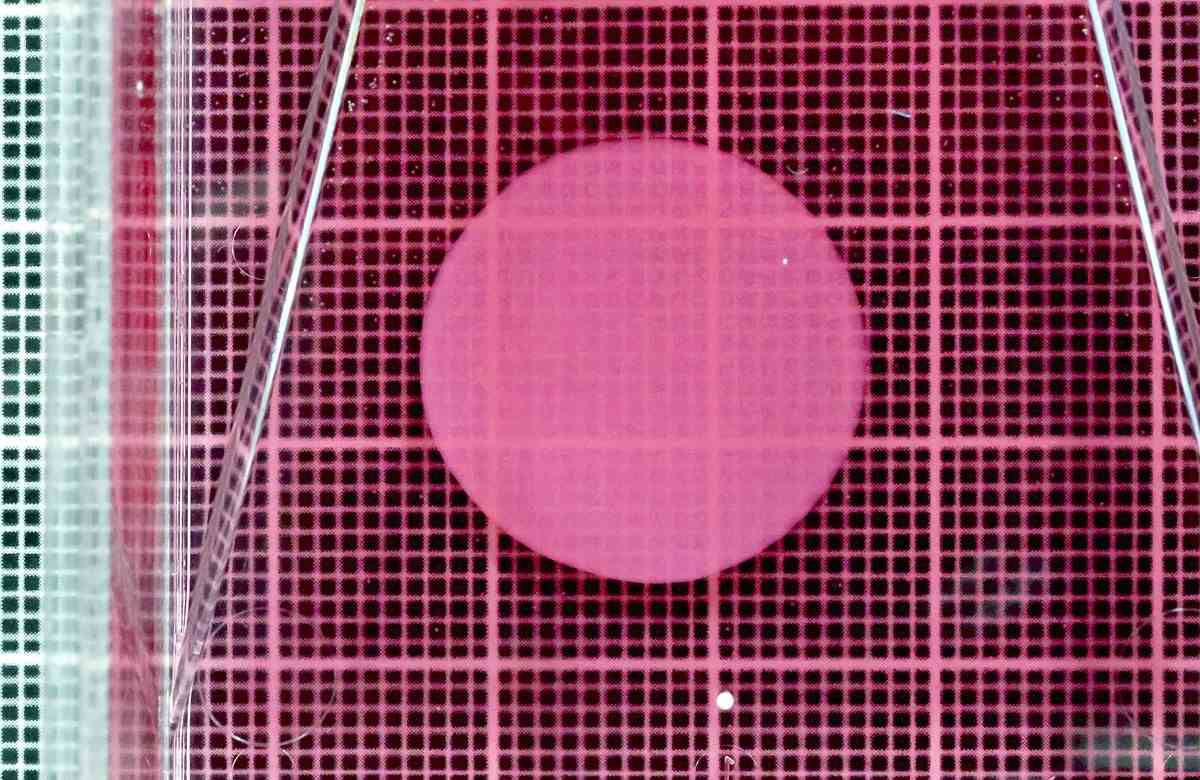
A heart muscle cell sheet made from iPS cells is shown in Suita, Osaka Prefecture, in April.
1:00 JST, May 28, 2024
A startup launched at Osaka University plans to apply soon for approval to commercially sell sheets of heart muscle cells made from induced pluripotent stem (iPS) cells, according to the company.
Tokyo-based Cuorips Inc. will apply with the Health, Labor and Welfare Ministry as early as June for permission to produce and sell the sheets, which are to be transplanted into the hearts of patients suffering from myocardial infarctions (heart attacks) and similar ailments.
It is likely that this will be the first ever use of a regenerative medical treatment product made from iPS cells. Hospitals may soon begin actively using medical products made from iPS cells.
The sheets are expected to be used to treat sufferers of ischemic heart diseases, in which the heart muscle is weakened by a heart attack or other cause.
If symptoms become serious, the patients will need to receive heart transplants. But the number of heart donors is small, and heart transplantation also severely taxes the receiving patient’s body.
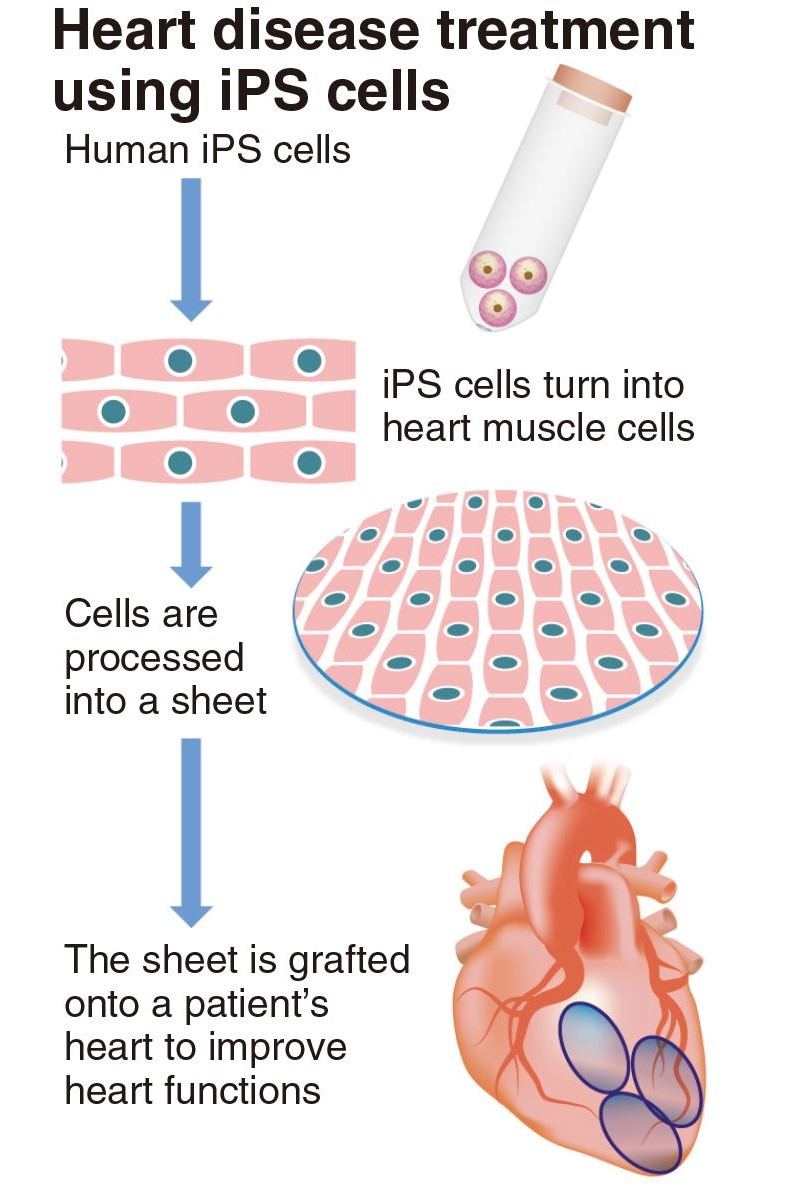
Yoshiki Sawa, a Distinguished Professor at Osaka University who also serves as the supreme technological manager of Cuorips, and his team produced heart muscle cells from human iPS cells, then processed them into the shape of sheets.
From January 2020 to March 2023, they conducted clinical tests on a total of eight patients with ischemic heart diseases. They grafted sheets of approximately 100 million cells onto each patient’s heart.
Sawa and his team said that the treatment was confirmed to be safe for eight patients and all of them were able to return to normal life.
If the company can estimate the product’s effectiveness and other qualities despite the small size of the clinical trial, they plan to make use of a system in which they would be approved to produce the product under certain conditions for a limited amount of time. The company aims to obtain this approval in 2025.
Competition to develop medical products using iPS cells is growing increasingly fierce worldwide.
In Japan, Osaka-based Sumitomo Pharma Co. aims to apply for approval of nerve cells derived from iPS cells, to be administered to Parkinson’s disease sufferers, as early as fiscal 2024. This and other research toward practical uses of iPS cells continue apace.
Top Articles in Science & Nature
-

Japan Institute to Use Domestic Commercial Optical Lattice Clock to Set Japan Standard Time
-

Japan to Face Shortfall of 3.39 Million Workers in AI, Robotics in 2040; Clerical Workers Seen to Be in Surplus
-

Record 700 Startups to Gather at SusHi Tech Tokyo in April; Event Will Center on Themes Like Artificial Intelligence and Robotics
-

iPS Treatments Pass Key Milestone, but Broader Applications Far from Guaranteed
-

iPS Cell Products for Parkinson’s, Heart Disease OK’d for Commercialization by Japan Health Ministry Panel
JN ACCESS RANKING
-

Japan PM Takaichi’s Cabinet Resigns en Masse
-

Japan Institute to Use Domestic Commercial Optical Lattice Clock to Set Japan Standard Time
-

Israeli Ambassador to Japan Speaks about Japan’s Role in the Reconstruction of Gaza
-

Man Infected with Measles Reportedly Dined at Restaurant in Tokyo Station
-

Videos Plagiarized, Reposted with False Subtitles Claiming ‘Ryukyu Belongs to China’; Anti-China False Information Also Posted in Japan