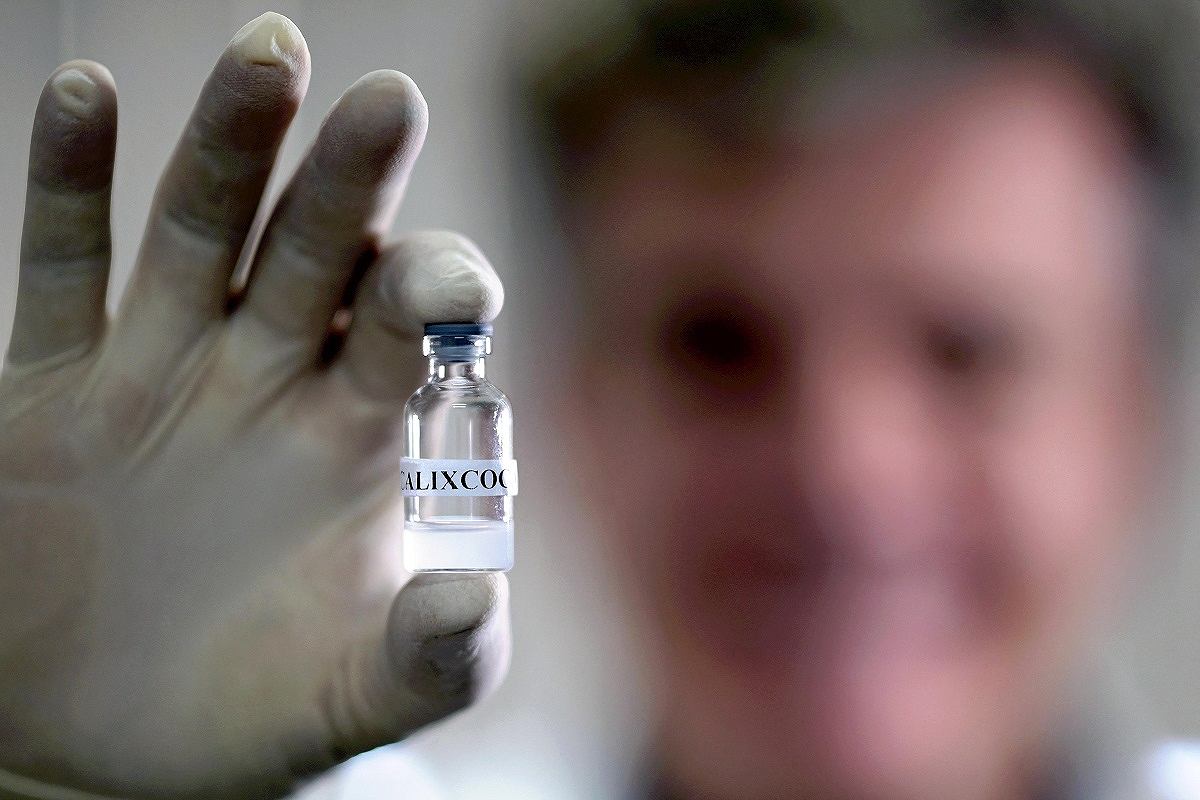
Brazilian psychiatrist Frederico Garcia holds a vial of a test vaccine for cocaine addiction at the Federal University of Minas Gerais, in Belo Horizonte, Brazil, on June 7.
15:34 JST, November 2, 2023
BELO HORIZONTE, Brazil (AFP-Jiji) — Scientists in Brazil, the world’s second-biggest consumer of cocaine, have announced the development of an innovative new treatment for addiction to the drug and its powerful derivative crack: a vaccine.
Dubbed “Calixcoca,” the test vaccine, which has shown promising results in trials on animals, triggers an immune response that blocks cocaine and crack from reaching the brain, which researchers hope will help users break the cycle of addiction.
Put simply, addicts would no longer get high from the drug.
If the treatment gets regulatory approval, it would be the first time cocaine addiction is treated using a vaccine, said psychiatrist Frederico Garcia, coordinator of the team that developed the treatment at the Federal University of Minas Gerais.
The project won top prize last month — €500,000 ($530,000) — at the Euro Health Innovation awards for Latin American medicine, sponsored by pharmaceutical firm Eurofarma.
The vaccine works by triggering patients’ immune systems to produce antibodies that bind to cocaine molecules in the bloodstream, making them too large to pass into the brain’s mesolimbic system, or “reward center,” where the drug normally stimulates high levels of pleasure-inducing dopamine.
Similar studies have been carried out in the United States — the world’s top cocaine consumer, according to the United Nations Office on Drugs and Crime. But they stalled when clinical trials did not demonstrate sufficient results, among other reasons, Garcia said.
Calixcoca has so far proven effective in testing on animals, producing significant levels of antibodies against cocaine and few side effects.
It also protected rat fetuses against cocaine, researchers found, suggesting it could be used in humans to protect the unborn babies of pregnant addicts.
The vaccine is now set to enter the final stage of trials: testing on humans.
No ‘panacea’
Garcia said Calixcoca could reshape addiction treatment.
“There’s no specific registered treatment for cocaine and crack addiction. We currently use a combination of psychological counseling, social assistance and rehabilitation, when necessary,” he said.
Calixcoca could add an important tool to that regimen, helping patients at critical stages of recovery, such as when they leave rehab, he said.
The vaccine is made with chemical compounds designed in the lab, rather than biological ingredients, meaning it would be less expensive to produce than many vaccines and would not have to be stored at cold temperatures.
But it won’t be a “panacea” that can be administered to anyone, Garcia said.
The exact target group will depend on the outcome of clinical trials, but is theoretically meant to be recovering addicts “who are off [cocaine] and want to stay that way,” he said.
The goal is to change what Garcia calls a “sad statistic.” According to the U.S. National Institute on Drug Abuse, one in four regular cocaine users become addicted.
And just one in four addicts manages to quit after five years of treatment.
Given the stakes, anticipation around the vaccine is high. More than 3,000 people have contacted Garcia’s team to volunteer to take part in the clinical trials.
Top Articles in Science & Nature
-

Japan Institute to Use Domestic Commercial Optical Lattice Clock to Set Japan Standard Time
-

Japan to Face Shortfall of 3.39 Million Workers in AI, Robotics in 2040; Clerical Workers Seen to Be in Surplus
-

Record 700 Startups to Gather at SusHi Tech Tokyo in April; Event Will Center on Themes Like Artificial Intelligence and Robotics
-

iPS Treatments Pass Key Milestone, but Broader Applications Far from Guaranteed
-

iPS Cell Products for Parkinson’s, Heart Disease OK’d for Commercialization by Japan Health Ministry Panel
JN ACCESS RANKING
-

Japan PM Takaichi’s Cabinet Resigns en Masse
-

Japan Institute to Use Domestic Commercial Optical Lattice Clock to Set Japan Standard Time
-

Israeli Ambassador to Japan Speaks about Japan’s Role in the Reconstruction of Gaza
-

Man Infected with Measles Reportedly Dined at Restaurant in Tokyo Station
-

Videos Plagiarized, Reposted with False Subtitles Claiming ‘Ryukyu Belongs to China’; Anti-China False Information Also Posted in Japan





















