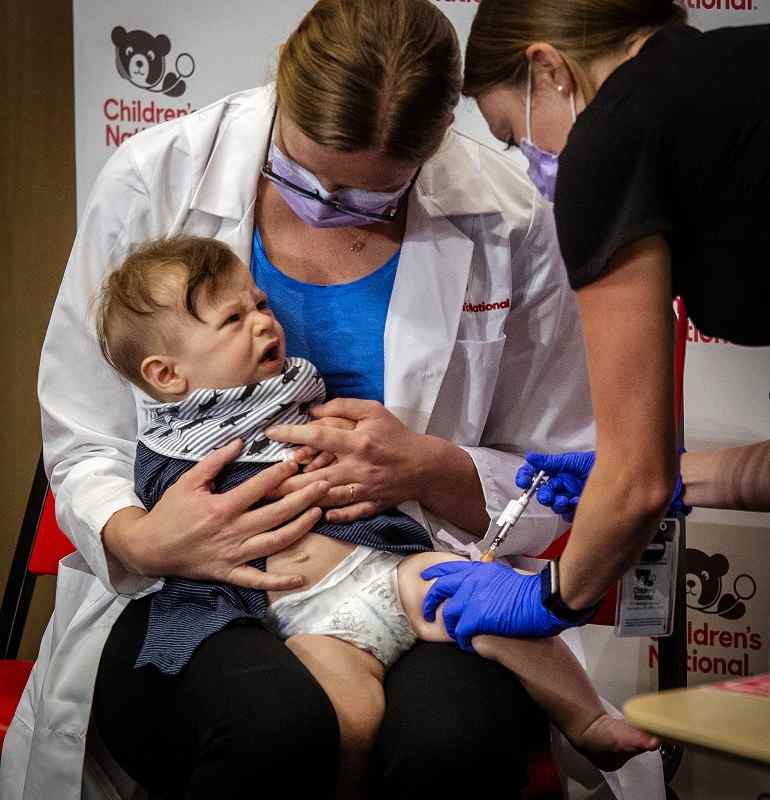
Physician Sarah Schaffer Deroo calms her 7 month-old-son, Hewitt, as he receives a coronavirus vaccine in D.C.
11:27 JST, December 9, 2022
Federal regulators Thursday authorized an updated booster shot of Moderna’s coronavirus vaccine for young children, saying the inoculation would offer increased protection amid a wave of respiratory illnesses that is increasing peril for youngsters. This means the youngest Americans will have access to variant-targeting boosters already available to older children and adults.
The Food and Drug Administration, in a statement, said children 6 months through 5 years old would be eligible for the booster – known as a “bivalent” shot targeting the omicron subvariants BA.4 and BA.5 and the original version of the virus – two months after they had completed Moderna’s two-dose primary series.
The agency also cleared an updated shot of the coronavirus vaccine by Pfizer and its German partner BioNTech for children 6 months through 4 years old. That bivalent shot, which also targets omicron, will be substituted for the last shot in the companies’ initial three-dose series. It is not considered a booster shot.
The FDA in June authorized a two-shot series of the Moderna vaccine of 25 micrograms each for young children, and a three-dose regimen of the Pfizer-BioNTech vaccine of 3 micrograms each. Pfizer and BioNTech have said they chose a lower dose to minimize potential side effects.
The Centers for Disease Control and Prevention is expected to sign off on the updated shots shortly.
The government’s latest effort to bolster protections is bumping up against an unwelcome fact: Only a small percentage of young children have been vaccinated against the coronavirus. Fewer than 5 percent of children 4 and younger have completed an initial series of a coronavirus vaccine, and fewer than 10 percent have received even a first shot, according to the CDC.
The FDA’s steps “won’t have a lot of real-world impact,” said Peter Hotez, a professor of pediatrics and molecular virology and microbiology at Baylor College of Medicine. “It’s like arguing over how many angels can dance on the head of a pin.”
But federal health officials, eyeing a rise in covid-19 cases and pediatric hospitalizations because of an array of respiratory germs, said it was important to make updated shots available to the youngest age groups.
Peter Marks, the FDA’s top vaccine regulator, said the agency wanted “parents who are trying to optimally protect their children” to have access to the most-up-to-date versions of the vaccine. He also said the authorizations give the agency another opportunity to stress that all children should be vaccinated against the coronavirus because it is the best way to prevent the worst outcomes, including hospitalization and death.
With temperatures dropping and activities moving indoors, the nation is “entering into another wave of covid,” Marks said. “We don’t know how high it is going to go.”
The BA.4 and BA.5 coronavirus subvariants, which were circulating widely this summer, have faded and are responsible for below 15 percent of cases, according to the CDC. They have been replaced by BQ.1 or BQ.1.1, which are causing more than 60 percent of cases in the United States.
Officials say the bivalent formulation will offer improved protection against the latest crop of omicron subvariants. Still, that shield is not foolproof: The latest mutants have proved adept at dodging some of the protective antibodies generated by even that shot.
Some vaccine experts are worried that the emergency authorizations could cause confusion among parents, especially those whose children have received the Pfizer-BioNTech vaccine. The bottom line is this: Children who have not started getting that vaccine’s three-dose series, or have received only two shots, will get the bivalent vaccine, not the original shot, as their third inoculation.
But children who have received the three-shot primary series of the Pfizer-BioNTech vaccine will not be eligible immediately for a booster. The FDA is expected to review data for a bivalent booster for the Pfizer-BioNTech shots early next year. These children “would still be expected to have protection against the most serious outcomes from the currently circulating omicron variant,” the FDA said.
Children’s hospitals in the United States are under strain as they care for unusually high numbers of children infected with RSV and other respiratory viruses. Doctors worry that the numbers will rise as families and friends gather for the holidays.
But the boosters have hardly been embraced, even by adults. The uptake of a bivalent booster has been lackluster, and the White House recently said it would launch a six-week push to increase booster rates among older people, in communities of color and in rural areas – all of which have suffered disproportionately high rates of severe disease and death during the coronavirus pandemic.
Top Articles in News Services
-

Survey Shows False Election Info Perceived as True
-

Prudential Life Expected to Face Inspection over Fraud
-

Hong Kong Ex-Publisher Jimmy Lai’s Sentence Raises International Outcry as China Defends It
-

Japan’s Nikkei Stock Average Touches 58,000 as Yen, Jgbs Rally on Election Fallout (UPDATE 1)
-

Japan’s Nikkei Stock Average Falls as US-Iran Tensions Unsettle Investors (UPDATE 1)
JN ACCESS RANKING
-

Japan PM Takaichi’s Cabinet Resigns en Masse
-

Japan Institute to Use Domestic Commercial Optical Lattice Clock to Set Japan Standard Time
-

Israeli Ambassador to Japan Speaks about Japan’s Role in the Reconstruction of Gaza
-

Man Infected with Measles Reportedly Dined at Restaurant in Tokyo Station
-

Videos Plagiarized, Reposted with False Subtitles Claiming ‘Ryukyu Belongs to China’; Anti-China False Information Also Posted in Japan