Japan Drugmaker Applies for Approval of Stem Cell-Derived Parkinson’s Treatment; Aims to Obtain Approval Within FY25
1:00 JST, August 9, 2025
Sumitomo Pharma Co., a major drugmaker, announced on Tuesday that it has applied to the Health, Labor and Welfare Ministry for approval to produce and sell a regenerative medical product for the treatment of Parkinson’s disease.
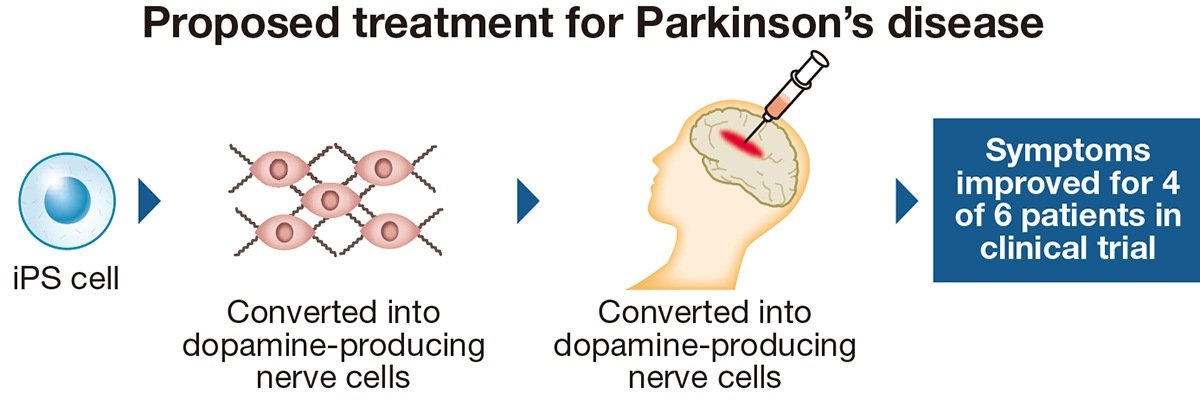
The drug contains dopamine-producing nerve cells derived from induced pluripotent stem (iPS) cells and is transplanted directly into patients’ brains.
It is the second application to the ministry for the approval of a regenerative medicine derived from iPS cells.
Parkinson’s is caused by a reduction in the nerve cells which produce dopamine, a neurotransmitter produced in the brain involved in body movement. Patients with the disease develop such symptoms as tremors in the hands or legs and have difficulty walking. In Japan, there are an estimated 250,000 people with Parkinson’s.

Sumitomo Pharma President Toru Kimura speaks of medicine derived from iPS cells on Wednesday in Tokyo.
The treatment method was jointly developed by Sumitomo Pharma and a team of researchers from Kyoto University. These researchers conducted clinical tests from 2018 to 2021. In the tests, 5 million to 10 million nerve cells were derived from iPS cells taken from healthy people and transplanted into the patients’ brains.
The researchers published the results in the science journal Nature in April this year, stating that four of the six patients experienced an improvement in their symptoms.
Sumitomo Pharma, which is in charge of producing the nerve cells, applied for the ministry’s approval based on the test results.
The regenerative medicine is covered by the government’s special screening designation system, in which priority is given to applications for revolutionary medical treatments originating in Japan. According to the ministry, the length of time necessary for the screening, which is usually about a year, will likely be shortened.
Sumitomo Pharma President Toru Kimura said, “We will make utmost efforts to obtain approval by the end of this fiscal year. We want to respond at maximum speed [for the ministry’s screening].”
The previous application for production and sales of a regenerative medicine derived from iPS cells was submitted by Cuorips Inc., an Osaka-based startup, in April. The startup makes sheets of heart muscle cells for patients with serious heart diseases.
Top Articles in Business
-

Prudential Life Insurance Plans to Fully Compensate for Damages Caused by Fraudulent Actions Without Waiting for Third-Party Committee Review
-

Narita Airport, Startup in Japan Demonstrate Machine to Compress Clothes for Tourists to Prevent People from Abandoning Suitcases
-

Japan, U.S. Name 3 Inaugural Investment Projects; Reached Agreement After Considerable Difficulty
-

Toyota Motor Group Firm to Sell Clean Energy Greenhouses for Strawberries
-

SoftBank Launches AI Service for Call Centers That Converts Harsh Customer Voices into Softer Voices
JN ACCESS RANKING
-

Japan PM Takaichi’s Cabinet Resigns en Masse
-

Japan Institute to Use Domestic Commercial Optical Lattice Clock to Set Japan Standard Time
-

Israeli Ambassador to Japan Speaks about Japan’s Role in the Reconstruction of Gaza
-

Man Infected with Measles Reportedly Dined at Restaurant in Tokyo Station
-

Videos Plagiarized, Reposted with False Subtitles Claiming ‘Ryukyu Belongs to China’; Anti-China False Information Also Posted in Japan





















