15:06 JST, January 30, 2026
Eleven medical facilities across the nation will soon start a clinical study that will examine all chromosomes of a fetus, in a move that could have implications for Japan’s current prenatal testing system that checks only three chromosomes for abnormalities.
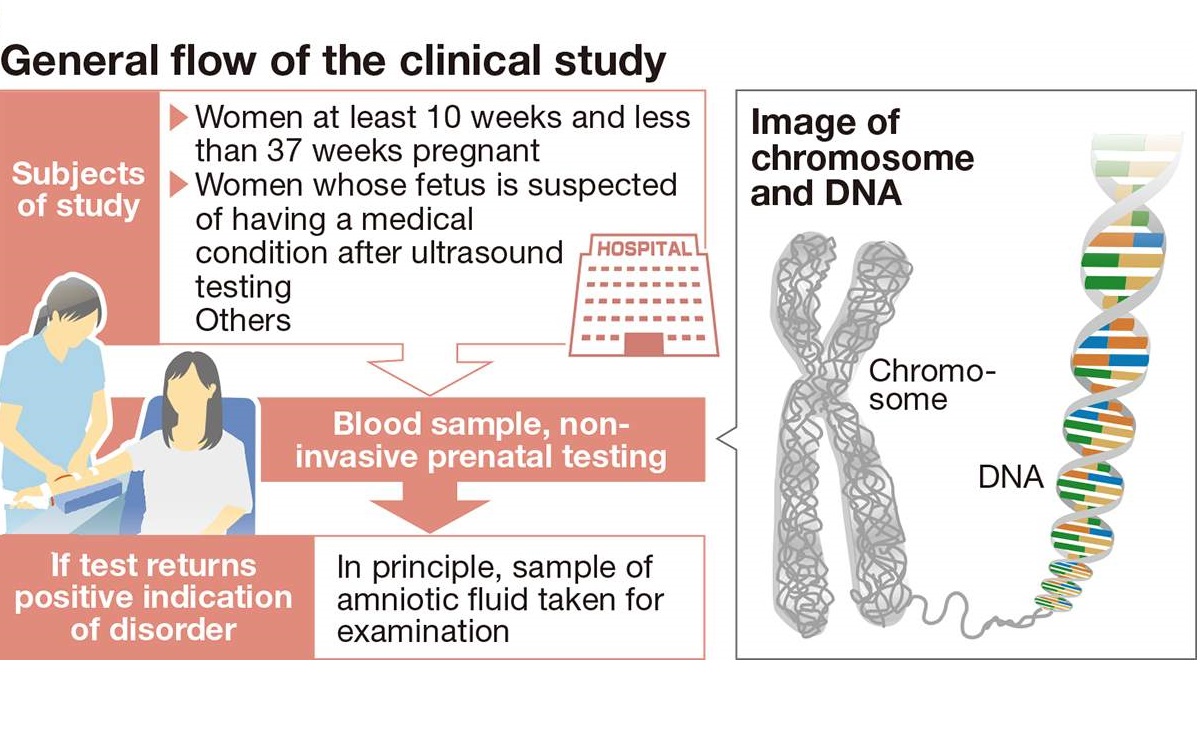
The study, which will involve non-invasive prenatal testing (NIPT), could start as soon as February. It will be conducted on about 2,000 pregnant women whose unborn babies are suspected of having chromosomal conditions, and verify the accuracy of these tests.
Humans typically have 23 pairs of chromosomes for a total of 46 chromosomes. If there are three or one chromosomes instead of two, or if a chromosome has parts missing or is duplicated, it can lead to congenital heart defects, delayed development and other health issues.
An NIPT involves taking a blood sample from a woman who is at least nine weeks pregnant, and checking the fetus’ DNA contained in her blood for possible chromosomal disorders. This testing was introduced in Japan in April 2013 and has been restricted to detecting abnormalities in chromosome 21 (which causes Down syndrome), chromosome 18 (Edwards syndrome) and chromosome 13 (Patau syndrome). These highly accurate tests are conducted at about 600 medical institutions approved by the Japanese Association of Medical Sciences’ steering committee.
University hospitals including Jikei University Hospital and other medical facilities approved by the committee will participate in the upcoming study, which will examine chromosome numbers and whether any chromosomes are missing or duplicated. The study will focus on women aged 18 or older and who are at least 10 weeks and up to 37 weeks pregnant, who have previously given birth to children with chromosomal disorders or whose fetus is suspected to have a health condition after undergoing an ultrasound and other tests. Women will be notified of the test results if they wish to be told.
If the test detects a chromosomal disorder, the diagnosis will be confirmed, in principle, by examining a sample of amniotic fluid collected by inserting a needle through the women’s abdomen into the uterus.
The study is scheduled to be conducted until the end of March 2030. As well as verifying the NIPT’s accuracy, the project will clarify the conditions under which medical facilities can conduct these tests, and also set up the necessary support systems for women both before and after undergoing the tests, such as the provision of genetic counselling.
This study comes at a time when medical facilities have, without approval from the committee, been conducting tests intended to detect chromosomal conditions other than the three permitted.
In some cases, confirmation of a fetal health condition can force a difficult choice regarding the life of an unborn child, such as whether to have an induced abortion. Testing for chromosomal conditions other than the three permitted is not accurate enough. However, according to reports from medical facilities authorized to conduct the NIPT, some institutions that specialize in other fields, such as cosmetic surgery and orthopedic surgery, have been performing these tests. Facilities sending notifications that disorders have being detected by mail and then not responding to requests for consultations have been among the problems reported.
The study framework includes a team comprising experts in various fields such as obstetricians, gynecologists, pediatricians and genetic specialists. This team will examine and verify the results from multiple perspectives.
Top Articles in Society
-

JAL, ANA Cancel Flights During 3-day Holiday Weekend due to Blizzard
-

Record-Breaking Snow Cripples Public Transport in Hokkaido; 7,000 People Stay Overnight at New Chitose Airport
-

Australian Woman Dies After Mishap on Ski Lift in Nagano Prefecture
-

Foreign Snowboarder in Serious Condition After Hanging in Midair from Chairlift in Nagano Prefecture
-

Train Services in Tokyo Resume Following Power Outage That Suspended Yamanote, Keihin-Tohoku Lines (Update 4)
JN ACCESS RANKING
-

Univ. in Japan, Tokyo-Based Startup to Develop Satellite for Disaster Prevention Measures, Bears
-

JAL, ANA Cancel Flights During 3-day Holiday Weekend due to Blizzard
-

China Confirmed to Be Operating Drilling Vessel Near Japan-China Median Line
-

China Eyes Rare Earth Foothold in Malaysia to Maintain Dominance, Counter Japan, U.S.
-

Japan Institute to Use Domestic Commercial Optical Lattice Clock to Set Japan Standard Time