
Preparations are underway to produce a COVID-19 vaccine at a pharmaceutical plant in Ikeda, Gifu Prefecture, on Feb. 18.
15:38 JST, June 24, 2021
Progress is being made in the development of novel coronavirus vaccines by Japanese companies. Clinical trials are underway at four companies. Two other firms aim to start trials by autumn. However, the hurdles are high for early commercialization of the vaccines.
Crisis management
“It is extremely important to establish a system to develop and produce a vaccine in Japan, not only for the maintenance of public health but also for crisis management,” Prime Minister Yoshihide Suga said at a meeting of the government’s Headquarters for Healthcare Policy at the prime minister’s office on June 1.
In the fiscal 2020 supplementary budget, the government invested more than ¥300 billion for basic research, clinical trials and the development of a production system for novel coronavirus vaccines. Although the development of the vaccine has been slower than in other countries, three companies have advanced to the second stage of three-stage clinical trials.
The government has secured enough vaccines to provide two shots to each member of the entire population, under contracts with U.S. firms Pfizer Inc. and Moderna, Inc. Even though the efficacy rates are as high as about 95% for the two vaccines, how long they will remain effective is unknown. There is a possibility that the vaccines will need to be administered on a regular basis, like influenza vaccines.
The supply of imported COVID-19 vaccines could become unstable due to export restrictions and other reasons, so the future production of a domestically developed vaccine is considered a necessity. Shionogi & Co., which is conducting clinical trials of a vaccine, is preparing production lines at its new plant in Gifu Prefecture and other facilities. The company plans to complete a production system by the end of this year to produce vaccines for 30 million people annually.
Final stage
However, there are challenges to realizing the early commercialization of the vaccines. One is the third and final stage of clinical trials.
Tens of thousands of people, including those in developing countries, participated in the final phase of clinical trials conducted by the companies that took the lead in COVID-19 vaccine development to test the efficacy of the vaccine and a placebo. Now that inoculations with approved vaccines have started in Japan, it is difficult to secure a large number of participants for clinical trials in which they would receive a placebo or a vaccine under development whose effectiveness and safety are unknown.
Another challenge is the approval review process. The United States has a system that allows the use of vaccines before their final approval in emergency situations with a short review, and Pfizer’s vaccine was allowed to be used within about three weeks of its application. Japan has a special approval system that simplifies the review process based on the assumption that the applicant vaccine has been administered overseas, but even so it took two to three months to complete the review of the Pfizer vaccine.
The special approval system is not designed for domestic vaccines that have not been used for inoculations, and it normally takes about a year to review such a vaccine.
International rules
Clinical trials are a challenge common to all countries where vaccine development has been delayed. Currently, drug approval organizations of various countries, including Japan, and other bodies are discussing how to establish international indexes to determine the efficacy of vaccines in an effort to reduce the scale of clinical trials.
“We need to reach a conclusion as soon as possible if we are going to use domestically produced vaccines after the vaccines [from overseas] that the government has already secured,” a senior official of the Health, Labor and Welfare Ministry said.
But no decision has been made yet. The government plans to inform companies of the direction of the discussions at an early stage and allow them to use new rules as soon as they are in place.
The government will also finalize the direction for simplifying the review process by the end of this year. In the case of drugs for rare diseases, for example, there is a system to conditionally approve the use of the drugs before the data from third-stage clinical trials is submitted. But how to ensure the safety of vaccines to be given to a large number of healthy citizens needs to be addressed.
Tetsuo Nakayama, a specially appointed professor of clinical virology at Kitasato University, said it is extremely difficult to put the vaccine into practical use by the end of this year under the current circumstances. “It is necessary to simplify the clinical trial and approval process while ensuring that the public can be assured of the efficacy and safety of vaccines. The government needs to have a strong awareness of the need to protect people’s lives with vaccines,” he said.
More government support
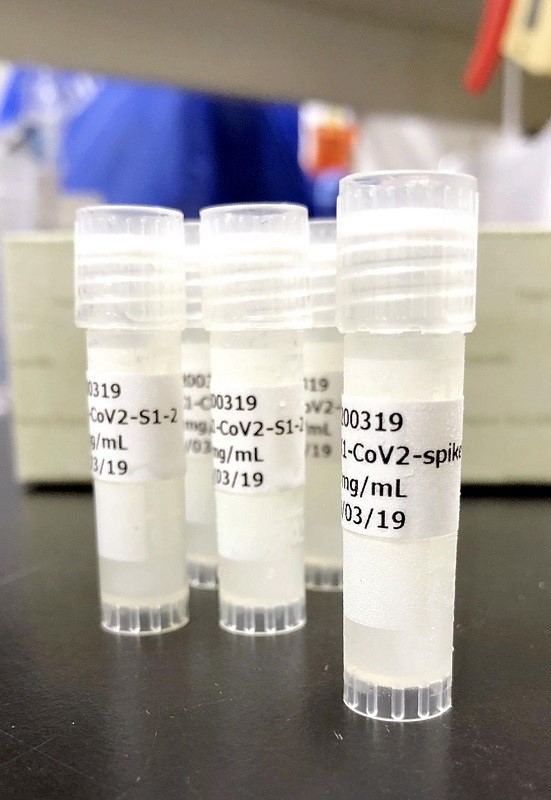
A COVID-19 vaccine for animal testing being jointly developed by medical start-up AnGes, Inc., Osaka University and others.
Historical circumstances lie behind the delay in the development of Japan-made vaccines. The responsibility of companies and the government was the subject of court cases in the 1970s and later as lawsuits were filed over harm caused by inoculations against smallpox and other diseases. These events fueled distrust of vaccination programs among the public, and Japanese companies became reluctant to develop high-risk vaccines.
Last year, the United States launched a campaign called Operation Warp Speed, investing about ¥2 trillion in vaccine development amid the spread of the coronavirus.
The early commercialization of the vaccine was largely due to earlier support measures, in which investments had been continuously made in vaccine development, placing importance on infection control measures for national defense reasons even before the breakout of the pandemic.
On June 1, the Japanese government approved a national strategy to strengthen vaccine development and production at a Cabinet meeting. It aims at creating and continuing to invest in a new organization in the Japan Agency for Medical Research and Development. The strategy also includes the creation of a system that will make it easier for companies to develop vaccines by allowing them to use vaccine production facilities for other biopharmaceutical production as well.
Still, concerns remain. In 2007, the government created a scheme called “vaccine industry vision” with similar purposes. An expert panel also compiled relevant proposals after the 2009-10 influenza epidemic. But neither of them was utilized.
In 2016, Ken Ishii, a professor of vaccine science at the Institute of Medical Science of the University of Tokyo, started developing an advanced vaccine with the government’s support, but the project was suspended in 2018 due to a lack of funds.
“This is the time to identify the causes of the failure and create a development system involving industry, government and academic organizations,” Ishii said.
Top Articles in Society
-

Man Infected with Measles Reportedly Dined at Restaurant in Tokyo Station
-
Man Infected with Measles May Have Come in Contact with Many People in Tokyo, Went to Store, Restaurant Around When Symptoms Emerged
-

Woman with Measles Visited Hospital in Tokyo Multiple Times Before Being Diagnosed with Disease
-

Australian Woman Dies After Mishap on Ski Lift in Nagano Prefecture
-

Foreign Snowboarder in Serious Condition After Hanging in Midair from Chairlift in Nagano Prefecture
JN ACCESS RANKING
-

Japan PM Takaichi’s Cabinet Resigns en Masse
-

Japan Institute to Use Domestic Commercial Optical Lattice Clock to Set Japan Standard Time
-

Israeli Ambassador to Japan Speaks about Japan’s Role in the Reconstruction of Gaza
-

Man Infected with Measles Reportedly Dined at Restaurant in Tokyo Station
-

Videos Plagiarized, Reposted with False Subtitles Claiming ‘Ryukyu Belongs to China’; Anti-China False Information Also Posted in Japan






















