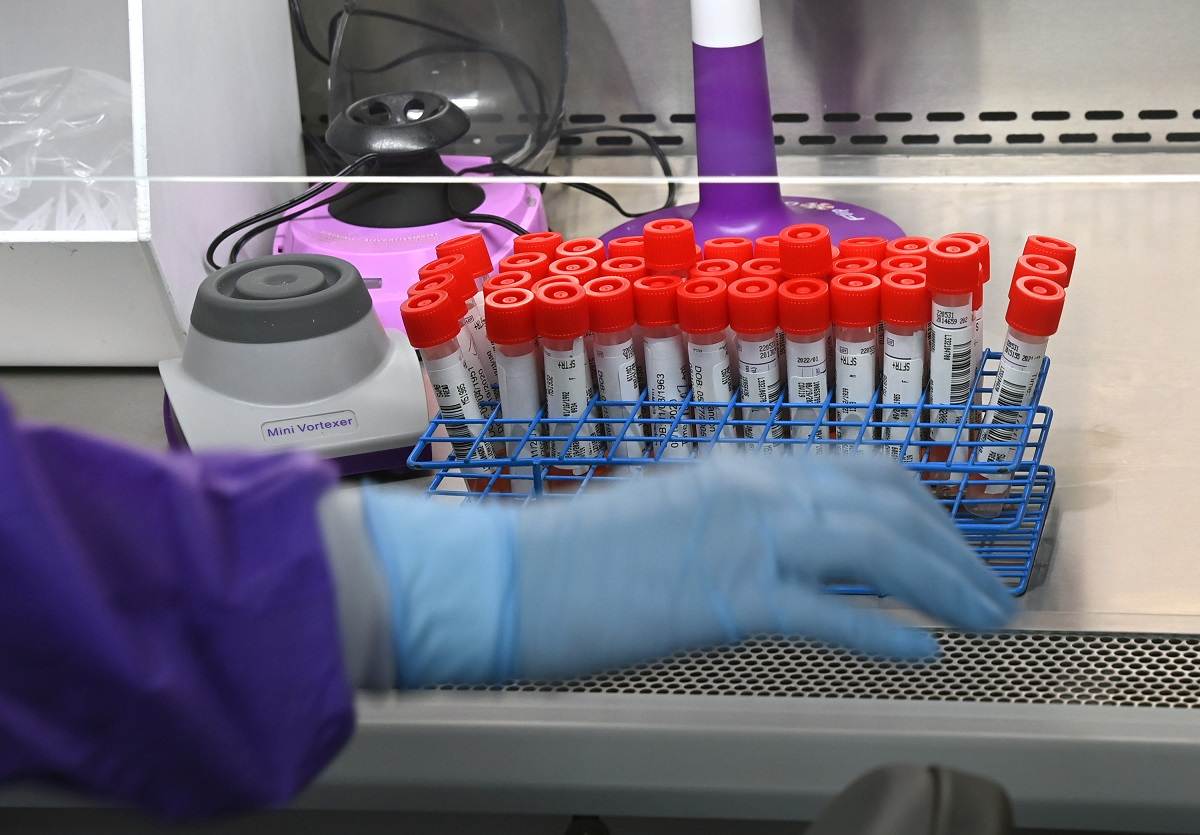
The Food and Drug Administration is seeking to regulate laboratory developed tests.
13:04 JST, April 30, 2024
The Food and Drug Administration has finalized a divisive plan to regulate laboratory medical tests – including some used to diagnose cancer and Alzheimer’s disease – over concerns about reliability and risks to patients.
The agency says inaccurate results may force patients to unnecessarily start a new treatment or delay care. Federal officials maintain that granting the FDA greater oversight will help ensure the tests are safe and effective, but critics contend the move will stifle the quick development of new tests.
“The agency cannot stand by while Americans continue to rely on results of these tests without assurance that they work,” FDA Commissioner Robert M. Califf said in a news release.
The new rules finalized Monday center on the multibillion-dollar industry of laboratory developed tests, known as LDTs, which are designed, manufactured and analyzed in a single laboratory as a screening and diagnostic tool. Major industry players, including academic medical centers that develop their own tests and large commercial laboratories, opposed the plan to further regulate the medical tests when it was proposed in September. Some analysts have said they expect the opponents to sue the FDA to prevent the new rules from going into effect.
“The rule will limit access to scores of critical tests, increase health care costs and undermine innovation in new diagnostics,” Susan Van Meter, president of the American Clinical Laboratory Association, a trade group with members including clinical lab giants LabCorp and Quest Diagnostics, said in a statement. The group has argued that such tests aren’t medical devices, like pacemakers or stents, and should not be regulated in a similar fashion.
The FDA does not review most lab tests before patients use them. But it finalized a plan to phase in the regulation of lab tests over the course of four years. Many tests will now have to go through premarket review from the FDA as well as adhere to other requirements, like the reporting of adverse events.
The agency is allowing tests already in use to stay on the market without necessarily going through the FDA’s new rules – a move the agency says is intended to address the risk that the cost of compliance could lead to the loss of tests patients rely on. Shares of Quest Diagnostics and LabCorp rose 3.9 percent and 2.9 percent, respectively, by the end of market trading Monday.
The rule “clearly showed FDA was trying to make sure not to upset the market,” said Michael Cherny, a senior managing director at Leerink Partners, an investment bank focused on health care and life sciences. “It encourages the appropriate deployment and development of lab-developed tests going forward by allowing and ensuring a pathway for these to get approved.”
Additionally, new tests used by hospital systems won’t need agency sign off if they address an unmet need, an aspect of the final rule that hospital lobbyists said they appreciated.
“However, we remain concerned that many vital tests developed in hospitals and health systems will still get caught up in unnecessary and costly paperwork,” Stacey Hughes, an executive vice president with the American Hospital Association, said in a statement.
Laboratory developed tests can be used to measure or detect a wide array of substances or markers in the body, such as proteins, glucose, cholesterol and DNA – all of which provide critical information about a patient’s health.
Inaccurate results can have a significant and deleterious impact on the decisions patients make. For instance, in 2022, the agency warned of the risk of false results from a prenatal screening test commonly used to provide information about the possibility of a fetus having a genetic abnormality, such as Down syndrome; the FDA said it is aware of reports of women ending pregnancies based solely on the test.
Decades ago, LDTs were much less common. But the prevalence of such tests has grown substantially with the ability to ship samples of patients’ blood and saliva to labs around the country, the FDA said.
The rule received support from watchdog groups and some nonprofits, including Friends of Cancer Research, whose president and chief executive, Jeff Allen, said it “sets forth a uniform approach to test regulation regardless of where a test is developed.”
But some consultants criticized the FDA rule as impractical. Jeffrey Gibbs, who advises laboratories and medical-device makers, said the new rule “raises questions about where the FDA will get the resources to take on this additional work.”
The issue has been in the crosshairs of lawmakers on Capitol Hill for several years. Congress has failed to pass bipartisan legislation solidifying the agency’s power. The lack of guidance prompted the agency to issue its own rules.
Some lawmakers have been critical of the rule, while others have said it is a necessary step to help drive patients’ decisions about their care.
“We are disappointed that the FDA has moved ahead with a burdensome rule,” Reps. Diana DeGette (D-Colo.) and Larry Bucshon (R-Ind.) said in a statement. The pair called on lawmakers to pass a bill they introduced to update oversight of diagnostic tests.
The FDA is open to working with Congress as well, said Jeff Shuren, the director of the FDA’s Center for Devices and Radiological Health.
Top Articles in News Services
-

Survey Shows False Election Info Perceived as True
-

Hong Kong Ex-Publisher Jimmy Lai’s Sentence Raises International Outcry as China Defends It
-

Japan’s Nikkei Stock Average Touches 58,000 as Yen, Jgbs Rally on Election Fallout (UPDATE 1)
-

Japan’s Nikkei Stock Average Falls as US-Iran Tensions Unsettle Investors (UPDATE 1)
-

Trump Names Former Federal Reserve Governor Warsh as the Next Fed Chair, Replacing Powell
JN ACCESS RANKING
-

Producer Behind Pop Group XG Arrested for Cocaine Possession
-

Japan PM Takaichi’s Cabinet Resigns en Masse
-

Man Infected with Measles Reportedly Dined at Restaurant in Tokyo Station
-

Israeli Ambassador to Japan Speaks about Japan’s Role in the Reconstruction of Gaza
-

Videos Plagiarized, Reposted with False Subtitles Claiming ‘Ryukyu Belongs to China’; Anti-China False Information Also Posted in Japan



























