21:00 JST, March 26, 2023
A team of researchers from Niigata University and the University of Tokyo will begin clinical tests this year of medicines to treat people with familial Alzheimer’s disease.
The tests will aim to remove abnormal proteins inside the brain that are believed to cause Alzheimer’s. The subjects will be people from families that have a tendency to develop hereditary dementia, which can present symptoms while the patient is still young.
The plan was examined by the two universities’ review boards for clinical tests in late February. Niigata University has given its approval, while the University of Tokyo has approved the plan with the condition that relevant documents will be revised.
The two Japanese universities are preparing to conduct the tests as part of joint international clinical tests in 16 countries and regions.
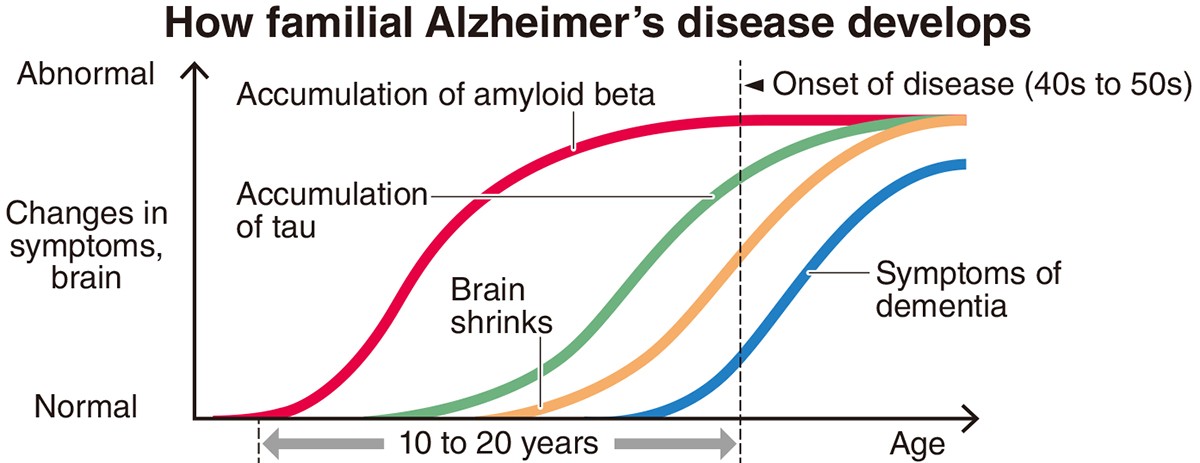
Alzheimer’s disease is believed to be caused by the gradual accumulation of proteins called amyloid beta and tau inside the brain. The buildup of these proteins can damage nerve cells, causing the brain to shrink. As a result, people with the disease suffer from impaired memory and judgment.
Scientists believe that the accumulation of amyloid beta begins 10 to 20 years before symptoms appear, and that the accumulation of tau starts after amyloid beta.
In many cases of familial Alzheimer’s disease, patients develop symptoms while relatively young — in their 40s to 50s. Accumulation of amyloid beta begins when they are in their 20s to 30s.
Past research has found that if people inherit such genetic mutations from their parents, they develop the symptoms at almost the same age as their parents.
Studying familial Alzheimer’s disease is therefore expected to help clarify the mechanism of the general type of Alzheimer’s disease and aid in the development of methods to treat it. As Japan’s population grays, the number of people suffering from the general type of Alzheimer’s disease has been on the rise.
The subjects of the clinical tests will be chosen from among people who are taking part in observation studies of familial Alzheimer’s disease. The subjects will be people who have been confirmed through blood checks to have the genetic mutations, and their ages will range from 10 years before the age at which the disease is estimated to develop to 10 years after developing the disease.
The subjects will include people with mild symptoms and people without symptoms.
All the subjects will receive intravenous drips of Lecanemab, a medicine to remove amyloid beta, for three to four years. The medicine was developed by major Japanese pharmaceutical maker Eisai Co. and its partners.
Lecanemab was swiftly approved for use in the United States in January for treating patients in early stages of Alzheimer’s disease. It is currently being screened in Japan.
Half of the test subjects will also receive intravenous drips of another medicine to prevent tau from spreading inside their brains. This medicine, called E2814, is being developed by Eisai.
The clinical tests will check for changes in the quantity of amyloid beta and tau inside subjects’ brains, and confirm whether the medicines can help prevent or slow the decline of cognitive function. The tests will also confirm whether there are differences between the group of subjects who receive only Lecanemab and those who receive both medicines.
Since last year, clinical tests have been conducted in the United States in which people who belong to a family with the possibility of developing familial Alzheimer’s disease receive treatment medicines
About 170 people worldwide, including 10 to 20 in Japan, are expected to participate in the clinical tests.
“As patients with familial Alzheimer’s disease often develop the disease at the peak of their work careers, there is a stronger desire for treatment,” said Prof. Takeshi Ikeuchi of Niigata University’s Brain Research Institute, who is responsible for the clinical tests in Japan. “It will be significant if the medicines are proven effective. Positive results can also be utilized for treating the general type of Alzheimer’s disease.”
Familial Alzheimer’s disease is caused by genetic mutations. Scientists have found three kinds of genetic mutations that cause this type of Alzheimer’s, and people have a 50% chance of inheriting the genetic mutations from their parents.
A nationwide survey in fiscal 2013 among doctors specializing in dementia confirmed the existence of 987 patients in 434 family lines.
Top Articles in Society
-

Producer Behind Pop Group XG Arrested for Cocaine Possession
-

Man Infected with Measles Reportedly Dined at Restaurant in Tokyo Station
-
Man Infected with Measles May Have Come in Contact with Many People in Tokyo, Went to Store, Restaurant Around When Symptoms Emerged
-

Woman with Measles Visited Hospital in Tokyo Multiple Times Before Being Diagnosed with Disease
-

Australian Woman Dies After Mishap on Ski Lift in Nagano Prefecture
JN ACCESS RANKING
-

Producer Behind Pop Group XG Arrested for Cocaine Possession
-

Japan PM Takaichi’s Cabinet Resigns en Masse
-

Man Infected with Measles Reportedly Dined at Restaurant in Tokyo Station
-

Israeli Ambassador to Japan Speaks about Japan’s Role in the Reconstruction of Gaza
-

Videos Plagiarized, Reposted with False Subtitles Claiming ‘Ryukyu Belongs to China’; Anti-China False Information Also Posted in Japan