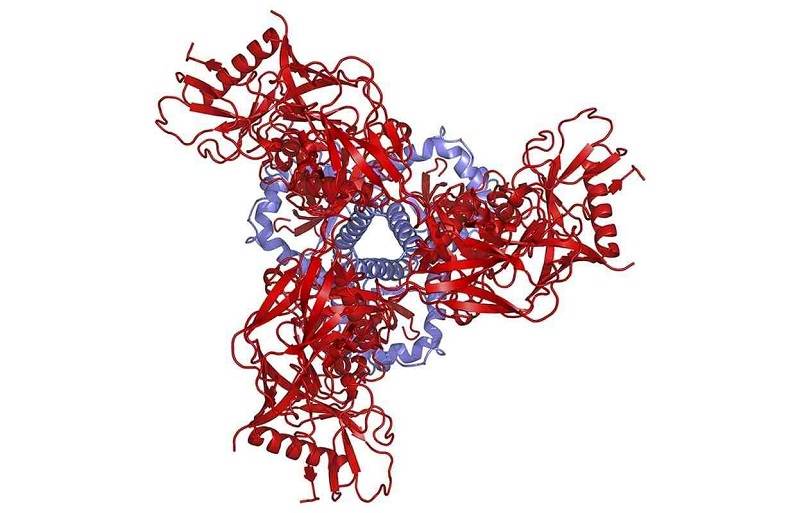
An artist illustration of a schematic depiction of the protein structure of the pre-fusion HIV spike as viewed from above shows the three gp41 molecules in blue and the three gp120 molecules in red, in this National Institute of Allergy and Infectious Diseases (NIAID) image released on October 8, 2014.
13:56 JST, June 2, 2022
Kathryn Stephenson was crushed last summer when she learned that an experimental HIV vaccine she had worked on for years failed to protect young women in sub-Saharan Africa from infection.
“I’m not afraid to say that I cried,” recalled Stephenson, a researcher at Beth Israel Deaconess Medical Center in Boston.
The failure wasn’t personal. Over decades, nearly every idea in science has been tried in the quest for an HIV vaccine – and faltered.
But the new technology that helped coronavirus shots break every speed record in medicine is opening a promising new avenue of research that could accelerate the pursuit of an HIV vaccine. Messenger RNA vaccines can be created and tested in months, not the year or more it can take for more traditional technologies.
Speed alone won’t solve the most difficult parts of the HIV problem. It will give scientists the ability to make and test vaccines quickly and establish a faster research rhythm: try ideas, tinker with them in real time and discard duds.
That agility is going to be crucial, because an HIV vaccine isn’t likely to be a shot, but a series of different shots, each tailored to nudge the immune system in the right direction, step by step.
The sheer technical challenges of creating, testing and refining an ornate sequence of shots had seemed a stretch to many experts – until the coronavirus pandemic proved that vaccine development could go lightning-fast.
“Even as recently as five years ago, there were a lot of people who would say this is a beautiful scientific idea but ridiculous to imagine in real life,” Stephenson said, describing the potential of mRNA vaccines. “I was one of those people. The world has changed.”
Despite years of effort, no HIV vaccine has coaxed the human immune system to churn out a protective force of highly specialized virus-fighting antibodies capable of blocking myriad versions of the virus. These broadly neutralizing antibodies, called bNAbs for short, have been a holy grail. But because it’s so hard to generate them, recent vaccination efforts have focused on other parts of the immune response, such as T cells or other types of antibodies.
Most HIV vaccine scientists agree: A protective vaccine will require bNAbs. To do that, scientists will have to solve some of the most difficult problems in the history of vaccinology.
‘A tall order’
In small human tests in clinics across the United States, a new generation of experimental HIV vaccines – powered by the same cutting-edge technology that brought leading coronavirus vaccines to the finish line in under a year – is being injected into people’s arms.
No one expects the same scientific triumph to unfold. HIV is a far more menacing foe than the coronavirus.
The emergence of coronavirus variants as a threat to vaccines pales, compared with the complexity and scale of the challenge posed by HIV variants. Often, so many variants exist in a single infected person that specialists don’t even count them, referring to a “swarm” of viruses. Also, HIV is cloaked in a shield of sugars that hide its vulnerable spots. And while the human immune system can clearly beat the coronavirus, the same is not true for HIV.
“HIV is the premier virus. It’s got more tricks on board than essentially any other virus,” said Dennis Burton, chair of the department of immunology and microbiology at Scripps Research Institute.
Vaccines work by presenting the immune system with a wanted poster – a telltale feature of a virus that immune warriors are supposed to hunt.
The coronavirus turned out to be easy prey. Coronavirus vaccines show the immune system the spikes that form a halo around the virus and elicit a force of virus-blocking antibodies in a matter of weeks.
HIV, in stark contrast, is devilishly hard to thwart.
Wanted posters quickly become obsolete as the virus mutates. HIV can also distract, hide from and confuse the immune system, focusing the body’s immune firepower on decoys. The immune cells capable of making a virus-blocking response are exceedingly rare. And unlike a coronavirus vaccine, which is considered a success even if a recipient develops a mild case of illness or asymptomatic infection, an HIV vaccine must block infection completely, because HIV can integrate into the body’s cells.
“It’s a tall order for a vaccine,” said Dan Barouch, a vaccine expert at Beth Israel Deaconess and an architect of the HIV vaccine that failed last summer. “It will have to act very fast, and either block infection – which may or may not be possible – or eliminate it exceedingly quickly, before it is able to seed a reservoir.”
Scientists believe that stopping HIV won’t involve a single wanted poster. Larry Corey, a virologist at the Fred Hutchinson Cancer Research Center in Seattle and a leader of a federally funded network that conducts human trials of HIV vaccines, compares the challenge in front of vaccine experts to raising a baby to be an elite athlete. Research right now, he said, is still at the infant-to-toddler transition.
“We’re now dealing with: How do you grow up that child?” Corey said. “There’s lots of ways to grow up.”
To do that, they’re going to have to try, fail, learn – and try again. That’s where messenger RNA comes in.
With traditional approaches, vaccines are brewed in a giant bioreactor and purified, a process that can take a year or longer. By the time one is ready to be tested in people, scientific thinking may have evolved.
With mRNA, creating a vaccine can be done in about three months. In the coronavirus pandemic, mRNA vaccines were first out of the gate, in late 2020. The first traditional protein vaccine in the United States could receive authorization next month, even as some people are already getting their second booster of the mRNA vaccines made by Moderna and Pfizer-BioNTech, and updated shots are being tested and prepared for the fall.
“If you want to do this iteration process and there’s a three-year gap between your idea and when you get into the clinic, it’ll always be the idea isn’t your best idea,” said Mark Feinberg, president of IAVI, a nonprofit research organization focused on developing vaccines for HIV and other infectious diseases.
Since the 1990s, scientists have studied how the antibodies known as bNAbs develop in some individuals with HIV over years of infection. The basic problem: A key part of the immune response includes B cells that generate virus-fighting antibodies. But B cells with the potential to churn out bNAbs against HIV are scarce, so the initial vaccination needs to find these outliers and nurture them. Then, follow-up vaccinations will help teach those B cells how to block many versions of the virus.
There are theories about how to do this, but there is nothing on the shelf ready to protect against HIV. To test and perfect that regimen, researchers have shifted to small “experimental medicine” trials with a few dozen study subjects, which allows rapid testing to determine whether the immune response appears headed in the right direction.
A more traditional approach would be to create the vaccine constructs and test them in 100 or 200 people to get a look at safety – a good approach if scientists are pretty sure their approach is right.
“If it’s not inducing the right kind of antibodies, it doesn’t help to do 150 people. You can get that out of 10 to 20 people,” said Barton Haynes, an immunologist at Duke University Medical Center in Durham, N.C. “Instead of taking a year and a half, it’s being done on the order of a few months.”
Clues to success
HIV researchers are clear-eyed about the challenges that lie ahead. “A lot of people say, ‘mRNA is not magic’ at meetings,” Stephenson said. But they also are optimistic – as the technology has matured, so has the scientific knowledge about how to make a successful vaccine.
“We kind of know, now, exactly what we need for an HIV vaccine. We haven’t known that until last year,” said Paul Goepfert, an infectious-diseases specialist at the University of Alabama at Birmingham.
Only one experimental HIV vaccine regimen has ever displayed a glimmer of promise. In 2009, a trial conducted in thousands of healthy men and women in Thailand was 30% protective. It was a point of hope, but a slim and contentious one that split the field. Some experts debated whether the effect was real.
In February 2020, as a novel respiratory virus ricocheted across the planet, an HIV vaccine trial seeking to confirm results from the Thai study was halted when it became clear the vaccine wasn’t working.
Then, as coronavirus variants dominated the news a year later, another blow came to HIV scientists – a trial testing a laboratory-brewed version of a bNAb failed to protect people. Then, in summer 2021, the shot on which Stephenson and colleagues had been working failed.
Those headlines buzzed by. They were simply new examples of a familiar storyline – more failures in the failure-filled odyssey of making an HIV vaccine.
They were the death knell for the idea that it would be possible to protect against HIV without bNAbs. But they also pointed the way to success.
In the antibody trial, people at risk of infection received infusions of laboratory-generated antibodies intended to protect them. Those infusions failed at their main mission, but a subset of people who were exposed to versions of HIV that were especially sensitive to the antibody had some protection. That suggested a cocktail of broadly protective antibodies might work. And it allowed researchers to calculate the exact level of antibodies that would be needed to afford protection.
“That’s a very high bar,” William Schief, an immunologist at Scripps Research Institute, said at the annual Conference on Retroviruses and Opportunistic Infections in February. “But at least we know what the bar is, and we can try and reach for that bar now.”
Experiments during the next five to 10 years, many scientists hope, will help guide researchers toward a vaccination regimen capable of protecting people. If they are successful, more challenges could lie ahead.
“The problem is, you could get a Pyrrhic victory – you could succeed in doing this, but if you require seven different immunizations, how is that going to go down in Soweto?” said John P. Moore, a professor of microbiology and immunology at Weill Cornell Medicine, referring to the township in Johannesburg. “You have to have, in the back of your mind, that you could succeed in such a complicated way that it wouldn’t be useful.”
Still, he and others agreed, they must try.
Top Articles in News Services
-

Prudential Life Expected to Face Inspection over Fraud
-

Hong Kong Ex-Publisher Jimmy Lai’s Sentence Raises International Outcry as China Defends It
-

Japan’s Nikkei Stock Average Touches 58,000 as Yen, Jgbs Rally on Election Fallout (UPDATE 1)
-

Trump Names Former Federal Reserve Governor Warsh as the Next Fed Chair, Replacing Powell
-

Suzuki Overtakes Nissan as Japan’s Third‑Largest Automaker in 2025
JN ACCESS RANKING
-

Japan Institute to Use Domestic Commercial Optical Lattice Clock to Set Japan Standard Time
-

Israeli Ambassador to Japan Speaks about Japan’s Role in the Reconstruction of Gaza
-
Man Infected with Measles May Have Come in Contact with Many People in Tokyo, Went to Store, Restaurant Around When Symptoms Emerged
-

Prudential Life Insurance Plans to Fully Compensate for Damages Caused by Fraudulent Actions Without Waiting for Third-Party Committee Review
-

Woman with Measles Visited Hospital in Tokyo Multiple Times Before Being Diagnosed with Disease